Introducing VSLS, reepot’s core technology
reepot is a medical device featuring ilooda’s VSLS® technology
equipped to a Nd:YAG laser source with a 532nm wavelength.
equipped to a Nd:YAG laser source with a 532nm wavelength.
Experience intense cooling and
the smoothness of sapphire
the smoothness of sapphire
VSLS® is reepot’s new energy model.
CPTL, is a method of
contact cooling technology which
contact cooling technology which
maintains a constant cooling temperature throughout treatment,
protecting the skin while facilitating a strong and stable energy output.
protecting the skin while facilitating a strong and stable energy output.



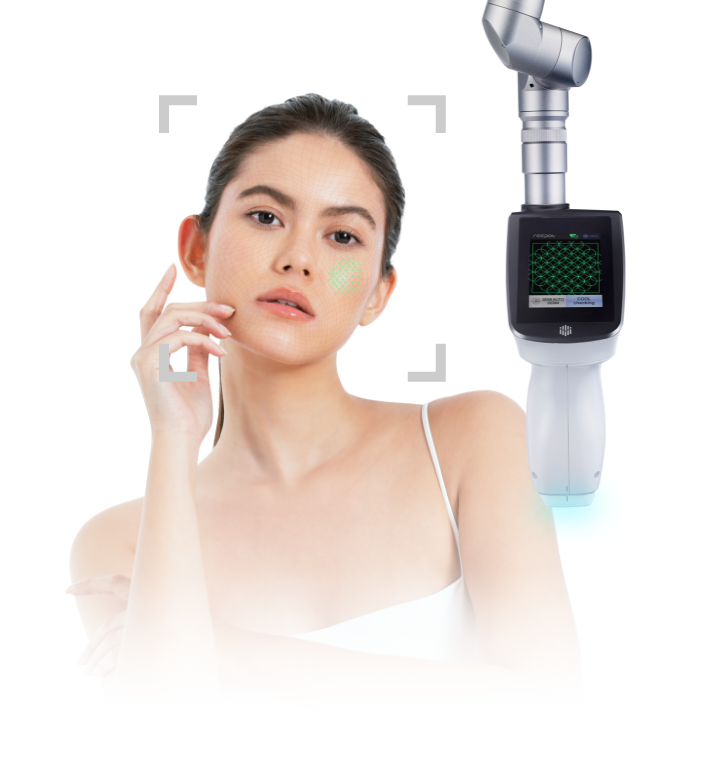
AutoDerm® is
a location detection and tracking guidance technology that ensures the laser’s energy is transmitted only at the target area.
The external medical camera facilitates safe treatment by employing real-time lesion data collection to adjust the energy transmitted to the target location.
The external medical camera facilitates safe treatment by employing real-time lesion data collection to adjust the energy transmitted to the target location.
Treatment Principles

*Image provided to assist with understanding
-
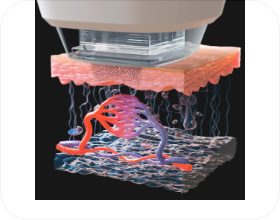
- STEP 01
- Target area captured
-
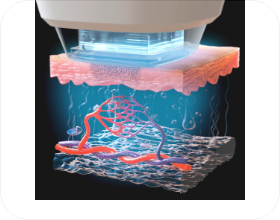
- STEP 02
- Hypercooling applied to target area
-
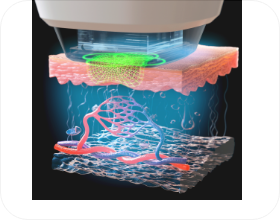
- STEP 03
- Image recognition of target area
-
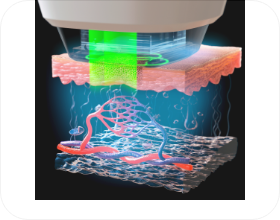
- STEP 04
- Laser output adjusted and
emitted to target area
Our Smart Glasses (HMD) were
designed with a crystal clear display to allow users to intuitively control the VSLS function
from any viewing angle and in any treatment setting.
from any viewing angle and in any treatment setting.

![]() The Smart Glasses are not a medical device.
The Smart Glasses are not a medical device.
ilooda holds American and Korean patients for the VSLS technology mechanism.
-


LIGHT TREATMENT DEVICE USING
LESION IMAGE ANALYSIS, METHOD OF
DETECTING LESION POSITION THROUGH
LESION IMAGE ANAL YSIS FOR USE
THEREIN,AND COMPUTING DEVICE
READABLE RECORDING MEDIUM HAVING
THE SAME RECORDED THEREINUS 10,525,276
-


A light treatment device using lesion image analysis, method of detecting lesion position through lesion image analysis for use therein, and computing device readable recording medium having the same recorded therein
KR 10-1580075
-


A light treatment device using lesion image analysis and handpiece used therein
KR 10-1351138


